TM 5-3895-359-14&P
scoring, piston seizure and cylinder head cracking are
the inevitable results. An improperly inhibited coolant
can also become corrosive enough to "eat away"
coolant passages and seal ring grooves and cause
coolant leaks to develop. If sufficient coolant
accumulates on top of a piston, a hydrostatic lock can
occur while the engine is being started. This, in turn,
can result in a bent connecting rod. An improperly
inhibited coolant can also contribute to cavitation
erosion. Cavitation erosion is caused by the collapse of
bubbles (vapor pockets) formed at the coolant side of an
engine component.
The collapse results from a
pressure differential in the liquid caused by the vibration
of the engine part. As bubbles collapse, they form pin
points of very high pressure. Over a period of time, the
rapid succession of millions of tiny bursting bubbles can
wear away (erode) internal engine surfaces.
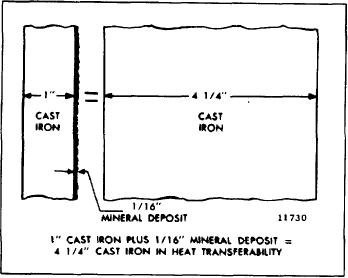
Fig. 1 - Heat Transfer Capacity
Components such as fresh water pump impellers and
cylinder liners are especially susceptible to cavitation
of these. Chlorides, sulfates, magnesium and calcium
erosion. In extreme cases their surfaces can become so
are among the materials which make up dissolved
deeply pitted that they appear to be spongy, and holes
solids. Water, within the limits specified in Table I is
can develop completely through them.
satisfactory as an engine coolant when proper
inhibitors are added. The procedure for evaluating
water intended for use in a coolant solution is shown in
Chromates
Table 2.
Sodium chromate and potassium dichromate are two of
the best and most commonly used water system
corrosion inhibitors.
Care should be exercised in
CORROSION INHIBITORS VITAL
handling these materials due to their toxic nature.
A corrosion inhibitor is a water-soluble chemical
compound which protects the metallic surfaces of the
Chromate inhibitors should not be used in antifreeze
cooling system against corrosive attack. Some of the
solutions.
Chromium hydroxide, commonly called
more commonly used corrosion inhibitors are
"green slime", can result from the use of chromate
chromates, borates, nitrates, nitrites and soluble oil.
inhibitors with antifreeze. This material deposits on the
cooling system passages and reduces the heat transfer
(Soluble oil is not recommended as a corrosion
rate (Fig. I) which results in engine overheating.
inhibitor). Depletion of all types of inhibitors occurs
Engines which have operated with a chromate-inhibited
through normal operation. Therefore, strength levels
water must be chemically cleaned before the addition of
must be maintained by the addition of inhibitors at
antifreeze. A commercial heavy duty descaler should
prescribed intervals.
be used in accordance with the manufacturer's
recommendation for this purpose.
The importance of a properly inhibited coolant cannot be
overstressed.
A coolant which has insufficient
inhibitors, the wrong inhibitors, or-worse-no inhibitors at
Soluble Oil
Soluble oil has been used as a corrosion inhibitor for
all invites the formation of rust and scale deposits within
many years. It has, however, required very close
the cooling system. Rust, scale, and mineral deposits
attention relative to the concentration level due to
can wear out water pump seals and coat the walls of the
adverse effects on heat transfer if the concentration
cylinder block water jackets and the outside walls of
exceeds 1% by volume. For example: 1.25% of soluble
the cylinder liners. As these deposits build up, they
oil in the cooling system increases fire deck
insulate the metal and reduce the rate of heat transfer.
temperatures 6% and a 2.50% concentration raises fire
For example, a 1/16" deposit of rust or scale on I" of
deck temperature up to 15%.
Soluble oil is not
cast iron is equivalent to 4-1/4" of cast iron in heat
recommended as a corrosion inhibitor.
transferability (Fig. I).
An engine affected in this manner overheats gradually
over a period of weeks or months. Liner scuffing,
10-9-14

